Intrauterine growth restriction (IUGR) remains one of the most vexing problems in modern obstetrics. Affecting roughly 5–7% of pregnancies, it is not simply a matter of having a “small baby.” Rather, it represents a pathophysiological failure in placental or fetal growth mechanisms, with consequences ranging from preterm birth and neonatal mortality to lifelong metabolic and neurodevelopmental sequelae. The search for interventions that can improve fetal outcomes has led researchers to examine therapies that enhance nitric oxide (NO) signaling, the central regulator of vascular relaxation in placental circulation. Two candidates—L-arginine and sildenafil citrate—have emerged as promising, if imperfect, tools in this effort.
This article explores the rationale, evidence, and limitations of these therapies, drawing on a meta-analysis that synthesized available clinical trials.
Understanding the Rationale: Nitric Oxide as the Placental Gatekeeper
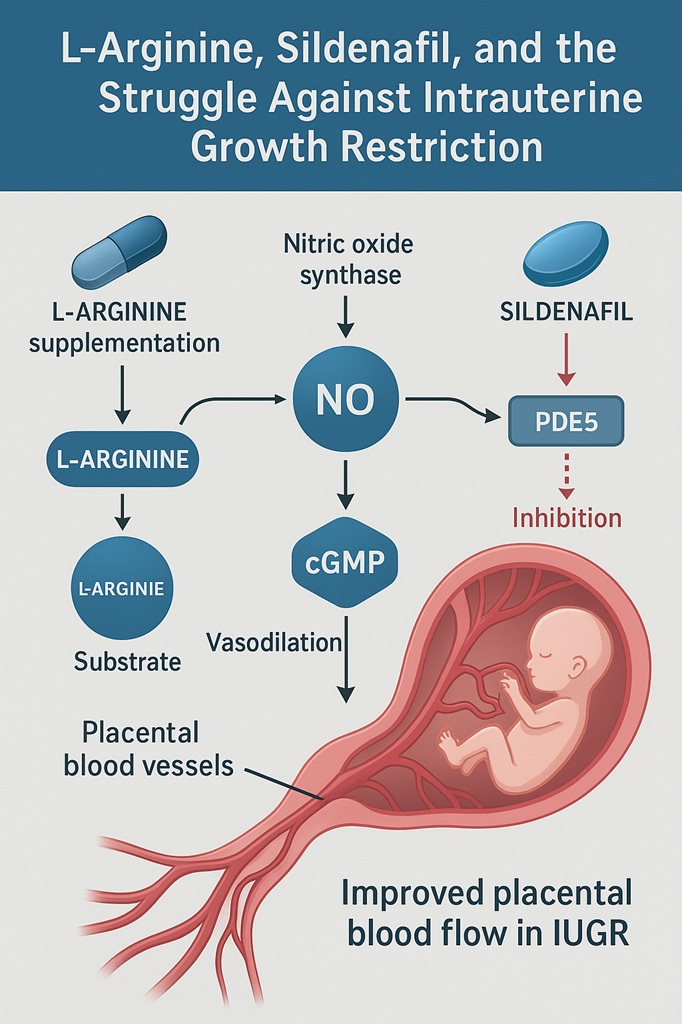
Nitric oxide (NO) is a deceptively simple gas that wields tremendous influence over vascular tone. Produced by endothelial nitric oxide synthase (eNOS) from the amino acid L-arginine, NO diffuses into vascular smooth muscle cells, triggering a cascade that elevates cyclic guanosine monophosphate (cGMP). Elevated cGMP levels relax vascular smooth muscle, dilating blood vessels and enhancing blood flow.
In pregnancy, this pathway is particularly critical. Adequate uteroplacental perfusion depends on NO-mediated remodeling of spiral arteries, ensuring that oxygen and nutrients flow to the fetus. When NO production falters—or when placental arteries fail to remodel properly—fetal growth suffers. In experimental models, pharmacological blockade of NO synthase induces IUGR, underscoring the centrality of this pathway.
This logic inspired two therapeutic strategies: supplementation with L-arginine (the substrate for NO synthesis) and inhibition of PDE5 by drugs like sildenafil, which prolong cGMP activity. Both approaches seek to restore placental blood flow, albeit via different biochemical steps.
L-Arginine: Substrate for Growth
Clinical Evidence
The meta-analysis reviewed nine randomized or quasi-randomized controlled trials involving 576 patients. Compared with placebo or no treatment, L-arginine supplementation consistently demonstrated:
- Increased birth weight: A standardized mean difference (SMD) of 0.41, representing meaningful weight gain in IUGR infants.
- Prolonged gestational age: An SMD of 0.30, suggesting that pregnancies were extended, reducing risks linked to prematurity.
- Reduced neonatal complications: Fewer cases of neonatal respiratory distress syndrome (NRDS) and intracranial hemorrhage (ICH), although these findings stemmed from small sample sizes.
Not all trials were uniformly positive. For example, in cohorts with severe, early-onset IUGR, benefits were muted, highlighting that timing and severity of pathology may dictate therapeutic success.
Mechanistic Insights
Beyond serving as a substrate for NO, L-arginine exerts ancillary effects:
- Promoting creatine and protein synthesis, supporting muscle growth.
- Enhancing growth hormone release.
- Stimulating polyamine synthesis, critical for placental development.
- Encouraging insulin secretion, a powerful fetal anabolic driver.
Thus, its benefits may extend beyond vascular dilation, contributing to systemic fetal growth support.
Sildenafil Citrate: Repurposing the Famous Blue Pill
Biological Mechanism
Sildenafil, better known for its role in erectile dysfunction, inhibits phosphodiesterase type 5 (PDE5), the enzyme that degrades cGMP. In the placenta, as in penile tissue, this prolongs NO’s vasodilatory effects, potentially improving oxygen and nutrient delivery to the fetus.
The irony is unmistakable: a drug designed to treat intimacy problems in aging men has been tested to nurture struggling fetuses in the womb. Biology, it seems, enjoys symmetry.
Clinical Evidence
Unfortunately, evidence for sildenafil in IUGR remains sparse. Only two small trials compared sildenafil with placebo or no intervention. Both demonstrated improved Doppler indices of umbilical and middle cerebral artery flow—important physiological markers. However, these studies were underpowered to detect meaningful differences in birth weight, survival, or long-term outcomes.
As such, the meta-analysis concluded that data were insufficient to determine sildenafil’s clinical role in IUGR management. Later large-scale trials, like the STRIDER studies, have since tempered enthusiasm, raising concerns about possible adverse neonatal outcomes.
Clinical and Practical Considerations
While L-arginine shows measurable benefit, several caveats remain:
- Bioavailability: Oral L-arginine is partly degraded in the gut and liver, limiting systemic availability. Intravenous administration bypasses this problem but is less practical.
- Heterogeneity of trials: Variations in dosage, duration, and patient selection complicate comparisons.
- Sample size limitations: For both L-arginine and sildenafil, most trials were small, limiting generalizability.
In practice, clinicians must balance these limitations against the paucity of alternatives. Currently, timely delivery remains the only definitive therapy for IUGR, making any intervention that can prolong gestation or enhance fetal growth highly valuable, even if modestly effective.
Looking Ahead: Research and Future Directions
The meta-analysis highlighted several critical gaps:
- Large, multicenter randomized controlled trials are needed to clarify the efficacy of both agents, especially sildenafil.
- Optimal dosing regimens for L-arginine require definition—oral vs. intravenous, duration of therapy, and patient selection criteria all remain unsettled.
- Long-term outcomes must be assessed, particularly neurodevelopmental trajectories in infants exposed to these therapies.
The broader lesson is that while repurposing existing drugs offers exciting opportunities, pregnancy presents a uniquely complex physiological context. Caution, rigorous study design, and long-term follow-up are essential before widespread clinical adoption.
Conclusion
L-arginine emerges as a promising adjunctive therapy for intrauterine growth restriction, with evidence supporting increased birth weight, prolonged gestation, and reduced neonatal complications. Sildenafil citrate, despite its mechanistic appeal, remains an experimental candidate, with current evidence too limited—and in some cases concerning—to justify routine use.
Both agents reflect the ingenuity of obstetric research in seeking solutions to IUGR, a condition where options are frustratingly scarce. For now, L-arginine appears more ready for cautious clinical use, while sildenafil’s role requires further clarification. In either case, the work underscores the need for continued innovation to improve outcomes for the most vulnerable of patients: fetuses struggling to grow before their first breath.
FAQ
1. Does L-arginine really help fetuses with intrauterine growth restriction?
Yes. Meta-analysis shows that L-arginine supplementation can increase birth weight and extend pregnancy duration, though results vary depending on severity and timing.
2. Is sildenafil a safe option for treating IUGR?
At present, no. While small trials showed improved blood flow, larger studies raised safety concerns, and evidence is insufficient to recommend routine use.
3. Why is nitric oxide so important in pregnancy?
Nitric oxide regulates uteroplacental blood flow by relaxing vascular smooth muscle. Reduced NO availability contributes to placental insufficiency and IUGR.
4. What remains the standard treatment for IUGR?
Careful monitoring and timely delivery. Pharmacological therapies like L-arginine and sildenafil remain investigational, used primarily in research settings.